Malaria’s new frontlines: vaccines, innovation, and the Indian endgame

In 2023, malaria contaminated almost 294 million individuals and killed shut to six,00,000. Regardless of early victories within the battle towards malaria, international progress has stalled in recent times. The parasites are adapting, changing into proof against therapies, whereas mosquitoes are surviving pesticides. It’s a shape-shifting enemy—and the previous instruments are slipping.
India has diminished its malaria burden by over 80% between 2015 and 2023—however final yr, tribal districts corresponding to Lawngtlai (Mizoram) and Narayanpur (Chhattisgarh) nonetheless recorded malaria charges of over 56 and 22 instances per 1,000 individuals, respectively as per the Nationwide Centre for Vector Borne Illnesses Management —reminders that the parasite continues to thrive in a number of pockets lengthy after nationwide averages have improved.
Whereas Africa faces principally Plasmodium falciparum, India additionally battles the relapse-prone Plasmodium vivax which may lie dormant within the liver and reawaken weeks or, even months later. In Jharkhand, combined infections account for almost 20% of instances (NCVBDC), complicating elimination. Even the place incidence has dropped, the parasite can persist—lurking in asymptomatic carriers (individuals with no signs) or returning months after an infection.
The seek for smarter, longer-lasting vaccines has by no means been extra pressing.
Hope with limits: RTS,S and R21
After a long time of setbacks, the primary permitted malaria vaccine—RTS,S—arrived in 2021. It provided about 55% safety within the first yr, however efficacy waned by 18 months, requiring a fourth booster dose.
The R21/Matrix-M vaccine, developed by Oxford and the Serum Institute, confirmed as much as 77% efficacy in Section 3 trials successful World Well being Group (WHO) approval in 2023. Fewer doses, low price, and Indian manufacturing make it particularly promising.
Nonetheless, each vaccines goal just one stage of the parasite, leaving reinfection and transmission a lingering risk.
Complete-parasite vaccines: a stronger shot on the horizon
As an alternative of focusing on a single protein, like in RTS,S and R21, whole-parasite vaccines expose the immune system to your complete malaria parasite—alive, however weakened. The experimental PfSPZ vaccine mimics pure an infection utilizing radiation-weakened P. falciparum sporozoite (the parasite’s early-stage type) delivered immediately into the bloodstream. Early research confirmed that 96% of members developed sturdy antibodies, with as much as 79% safety after the third dose.
Constructing on that basis, a modified model referred to as PfSPZ-LARC2, developed by Sanaria, could push efficacy even additional. The simplicity of a one-dose routine, regardless of the intravenous requirement, might make it a robust candidate for focused use in outbreak zones or amongst hard-to-reach migrant populations in India.
In contrast to vaccines that focus on the parasite’s earlier stage, PfRH5 acts through the blood stage, when signs seem and the danger of extreme sickness will increase. Since RH5 is a crucial protein for crimson blood cell invasion that the parasite can’t simply alter, it provides cross-strain safety—a uncommon asset in malaria vaccine design. Section 1a/b and Section 2b trials within the UK, The Gambia, and Burkina Faso have proven promising outcomes. These vaccines might complement earlier-stage ones and should assist enhance pure immunity in individuals who’ve beforehand had malaria.
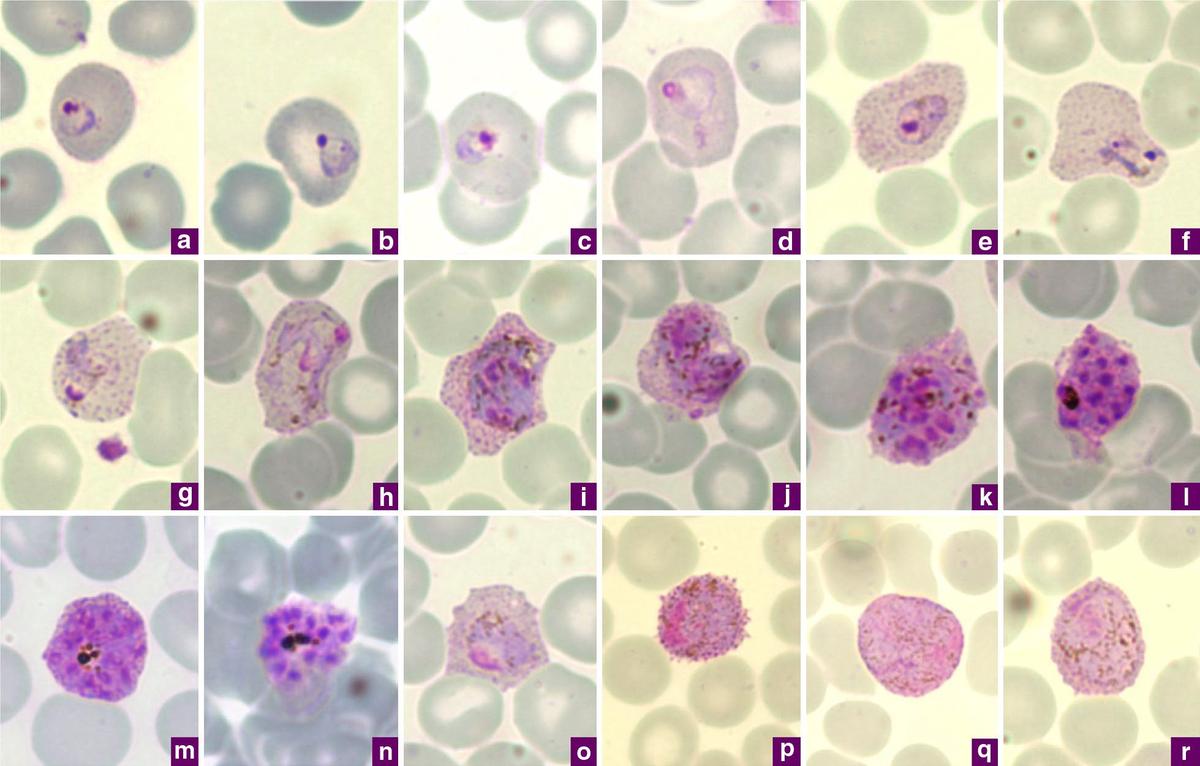
Photomicrographs of Plasmodium vivax in Giemsa-stained skinny blood movies
| Picture Credit score:
By Chavatte JM, Tan SB, Snounou G, Lin RT – Chavatte JM, Tan SB, Snounou G, Lin RT (2015). “Molecular characterization of misidentified Plasmodium ovale imported instances in Singapore.”. Malar J 14: 454. DOI:10.1186/s12936-015-0985-8. PMID 26577930. PMC: 4650842.- “This text is distributed beneath the phrases of the Inventive Commons Attribution 4.0 Worldwide License(https://creativecommons.org/licenses/by/4.0/)“, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=122024897
Transmission-blocking vaccines
Whereas the above vaccines intention to guard people, transmission-blocking vaccines (TBVs) goal the parasite within the mosquito—halting its unfold on the inhabitants stage. Pfs230D1 induces antibodies that stop parasite fertilization inside the mosquito intestine. In Mali, it diminished transmission by 78% in a Section 2 trial.
This technique is very related to India with a far larger proportion of asymptomatic carriers. “Our group and others in India are actively engaged on TBVs to handle this reservoir,” mentioned Agam P. Singh, scientist on the Nationwide Institute of Immunology, New Delhi.
India, too, is coming into the TBV area with its personal candidates. In July 2025, AdFalciVax was introduced by the Indian Council of Medical Analysis (ICMR), the nation’s first indigenous dual-stage malaria vaccine. In contrast to single-phase vaccines, it combines pre-erythrocytic (PfCSP) and transmission-blocking (Pfs230 and Pfs48/45) antigens to each stop an infection and block mosquito transmission. “AdFalciVax has accomplished preclinical testing,” mentioned Subhash Singh, who leads the programme at ICMR-RMRC Bhubaneswar. In mice, it triggered sturdy immune responses lasting over 4 months—roughly equal to a decade in people—and remained steady at room temperature for 9 months, doubtlessly aiding rural deployment.
Progress can also be seen past P. falciparum. A primary-in-human trial in Thailand confirmed that the P. vivax TBV Pvs230D1M diminished mosquito transmission by as much as 96%, one other ray of sunshine for India’s mixed-species numbers. India, too, just isn’t far behind. “An identical analysis program for P. vivax is underway, in collaboration with AdFalciVax co-inventors Sanghamitra Pati and Sushil Singh,” mentioned Dr. Singh.
Boosting immune energy
Strengthening the immune response itself is one other lively entrance. A latest protein-based vaccine mixed a ferritin nanoparticle with CpG—a sort of adjuvant, or immune booster already utilized in hepatitis B vaccines—and minimize liver-stage parasite burden by 95% in mice.
AdFalciVax confirmed over 90% safety in mice even with alum, a gentle and extensively used adjuvant. “We noticed safety on a par with extra inflammatory adjuvants corresponding to MPLA (a stronger adjuvant),” mentioned Dr. Singh. “Whether or not this holds in people stays to be seen.”
Scientists are additionally testing newer vaccine platforms corresponding to mRNA, which permit vaccines to be made sooner and tweaked extra simply than protein-based ones. In 2025, researchers at CureVac and the U.S. Nationwide Institute of Well being (NIH) encoded the Pfs25 antigen—focusing on the parasite’s sexual stage—into an mRNA-lipid nanoparticle. They noticed full transmission blockage in mice, with antibodies lasting over six months from simply two doses.
Nonetheless, not all mRNA-based vaccine efforts are transferring forward easily. In early 2025, BioNTech’s Section I/IIa trial for its blood-stage mRNA vaccine candidate BNT165e was positioned on medical maintain by the U.S. Meals and Drug Administration (FDA). Whereas the corporate didn’t disclose the explanation, it famous that discussions with regulators are ongoing. The pause highlights the hurdles of translating mRNA platforms into malaria vaccines.
“mRNA and nanoparticle platforms can actually be explored—alone or together,” mentioned Pawan Malhotra, emeritus scientist on the Worldwide Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi. “However it’s arduous to foretell what is going to work. Plasmodium is complicated, not like micro organism or viruses.”
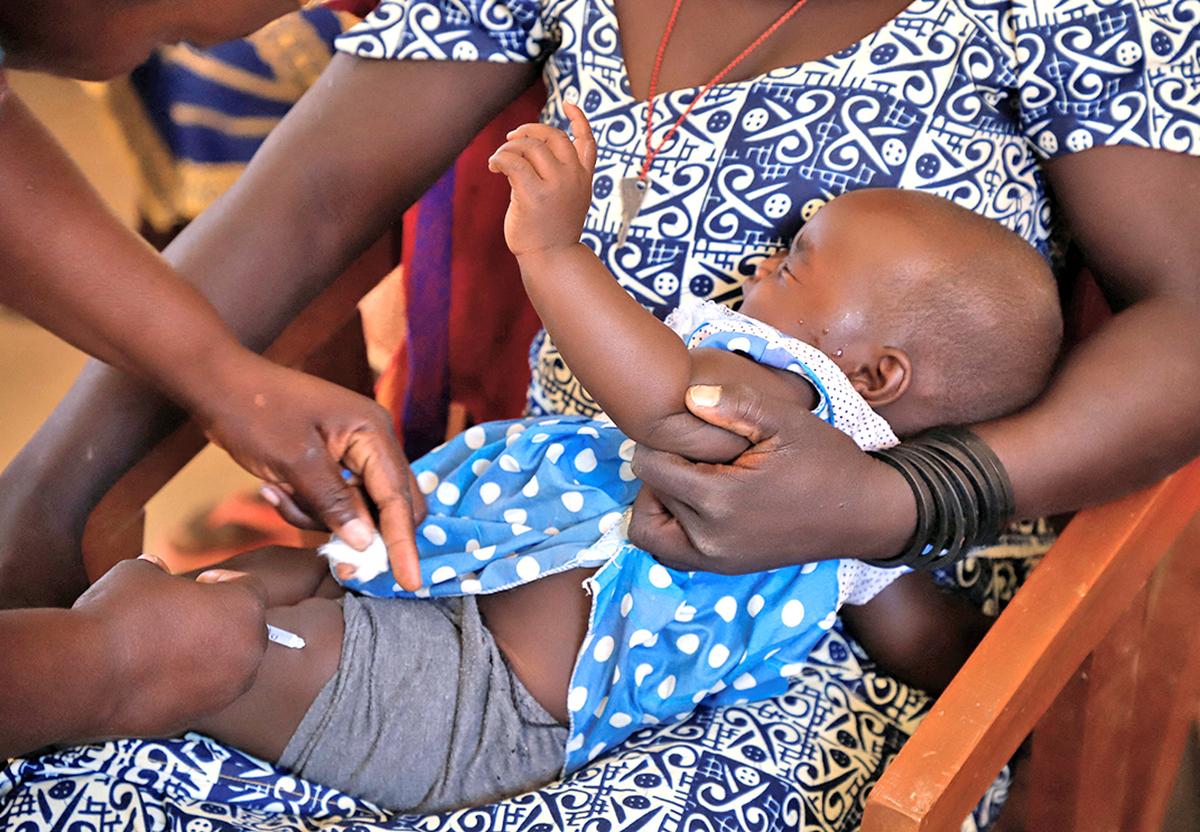
A nurse administers a malaria vaccine to an toddler on the well being middle in Datcheka, Cameroon January 22, 2024. File {photograph}
| Picture Credit score:
REUTERS
Augmenting current vaccines, blocking immune evasions
Past boosting the power of the immune response, scientists are additionally exploring tips on how to enhance its intention—modifying malaria antigens to assist the physique recognise the parasite extra effectively. A brand new experimental vaccine hyperlinks PfCSP—a floor protein from the malaria parasite—to MIP3α, a molecule that acts like a flare to attract in immune cells. In mice, it triggered stronger antibody and T cell responses than customary mRNA vaccines, lowering liver-stage an infection by as much as 88%. It hasn’t but been examined in people, but it surely exhibits how tweaking the immune response might push malaria vaccines previous present limits.
Past vaccines, researchers are exploring how malaria hides from our immune system. P. falciparum makes use of RIFIN proteins to bind to immune ‘off switches’ just like the LILRB1 receptor, shutting down immune cells.
It’s a tactic that helps the parasite cover in plain sight.
A brand new examine describes an experimental, engineered antibody, D1D2.v-IgG, designed to dam this interplay. Constructed from a section of the LILRB1 receptor, the antibody binds to RIFIN 110 occasions extra strongly than the pure model—outcompeting the parasite at its personal sport. By blocking this interference, it freed the physique’s LILRB1 to operate usually, restoring immune assault in lab assessments. Although nonetheless untested in animals, the strategy might sooner or later assist new malaria therapies or improve vaccine responses.
Gene drives and vector suppression
Whereas engineered antibodies assault the parasite, CRISPR-based gene drives go after its vector. These instruments insert fertility-disrupting genes into mosquitoes. In a landmark examine, this strategy worn out complete Anopheles gambiae colonies inside a yr—with no resistance detected.
However evolution not often performs alongside. Within the wild, mosquitoes would possibly adapt, ecosystems might shift, and as soon as launched, gene drives can’t be recalled. The concept of eradicating a species raises thorny moral and ecological questions.
So, researchers are exploring subtler methods. One 2025 examine edited a single letter within the FREP1 gene, blocking the malaria parasite from growing contained in the mosquito. With a gene drive, this parasite-blocking trait unfold to over 90% of lab mosquitoes in ten generations—with out harming their fertility or survival. However the parasite stays beneath stress to evolve across the block, and contaminated mosquitoes nonetheless dwell lengthy sufficient to doubtlessly transmit malaria if the trait doesn’t saturate the inhabitants.
One other staff took a unique route—engineering mosquitoes to die sooner solely when contaminated. By disabling an immune gene, they created a self-limiting suggestions loop: the extra malaria spreads, the extra it kills its personal carriers. As a result of this technique doesn’t assault the parasite immediately, it reduces the stress for resistance. It’s a sublime inversion—utilizing the parasite’s success towards itself to shrink transmission, with out eradicating the vector or requiring excellent protection.
The Indian lens: challenges and the trail forward
The nation goals malaria elimination by 2030. It’s an formidable plan—however one which hinges on precision and persistence.
Tribal and forested districts in Chattisgarh, Jharkhand and elements of the Northeast are the final strongholds of malaria—usually distant areas with restricted healthcare entry. Even in locations the place infections appear to be minimal, adults and older youngsters usually act as asymptomatic reservoirs, quietly sustaining transmission.
A municipal employee sweeps the street in entrance of a wall portray that depicts Sir Ronald Ross, identified for demonstrating that the Anopheles mosquitoes transmit the Plasmodium parasite, offering the primary evidence-based rationalization of malaria transmission
| Picture Credit score:
KVS GIRI
The twin-species panorama in India additional complicates elimination efforts.
“P. cynomolgi—a monkey malaria species—is the perfect mannequin for P. vivax analysis,” mentioned Dr. Malhotra. “We have been growing it 20 years in the past with the Central Drug Analysis Institute (CDRI), however strict monkey entry legal guidelines and lack of scientific foresight stalled it.”
Regardless of these challenges in vivax analysis, efforts to develop a vaccine are gaining floor. Each Dr. Subhash Singh at ICMR and Dr. Agam P. Singh at NII affirm that P. vivax vaccine candidates are beneath lively growth.
However even essentially the most progressive science wants programs to hold it ahead.
“We want a COVID-style push,” mentioned Dr. Malhotra. “Business and academia should collaborate with correct funding. We’ve developed potent therapeutic antibodies towards liver-stage parasites and now want partnerships to maneuver them ahead. A lone scientist in a lab can’t do all of it.” The science is advancing—but it surely wants infrastructure and political will to match.
Dr. Singh echoes the sentiment. “We at the moment are concentrating on translating AdFalciVax’s promising preclinical outcomes into trials. Profitable deployment nevertheless, would require good outcomes over a number of levels of trials in addition to regulatory approvals, possible taking not less than 7–8 years.” As well as, sturdy coordination between regulators, business, and researchers is required. ICMR has already floated an Expression of Curiosity looking for industrial companions to co-develop the vaccine. “Challenges that should be addressed embrace producing GMP-grade parts, growing immune biomarkers, and benchmarking efficacy towards RTS,S and R21,” added Dr. Singh.
“We undoubtedly want vaccines, antibodies, new medication—for each P. falciparum and P. vivax,” mentioned Dr. Malhotra. “However that’s not sufficient. Medical doctors want coaching, resistance should be tracked, and vector management has to maintain tempo.” It should be a full-spectrum battle—from the molecular stage to the group clinic.
India’s malaria story is now not one among uniform burden—it’s a battle towards hidden reservoirs, distant geographies, and a parasite that gained’t give up. With next-gen vaccines, homegrown innovation, and rising scientific momentum, the nation stands at a vital juncture. Elimination by 2030 isn’t just a aim—it’s a take a look at of whether or not science, coverage, and public well being can unite to defeat an historical foe. The instruments are right here. Now, we should use them—decisively and in every single place the parasite nonetheless survives.
(Anirban Mukhopadhyay is a geneticist by coaching and science communicator from Delhi. anirban.genetics@south.du.ac.in)



